The longer the non-polar part of alcohol C n H 2n-1 - hydrophobic the less soluble alcohol becomes. But insoluble in water 227 mg of CH 4 1 litre of water.

Solved Why Is Ethanol Ch3ch20h More Soluble In Water Than Chegg Com
And ethane only offers the possibility of weaker dispersion forces between molecules and has a boiling point of 89 C.
. Calculation of pH from Hydrogen Ion ConcentrationWhat is the pH of a solution that has an H concentration of a 175 10 5 molL. And the hydrogen attached to oxygen of ethanol makes a hydrogen bond with the oxygen of water. What makes something more soluble in ethanol.
When considering the chemical composition of two compounds ethane contains carbon and hydrogen atoms while ethanol has carbon hydrogen and oxygen atoms. If they do not dissolve in water those organic compounds are non-polar compounds. Properties of a Buffer The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments.
Sikringbp and 2 more users found this answer helpful. On the other hand ethyl alcohol has a boiling point of 78 C and this reflects the influence of hydrogen- bonding as a binding force between molecules. Ethanol is more soluble in water because the hydroxyl group on the end of alcohol forms hydrogen bonds with water molecules while ethane is hydrophobic and causes the solution to be highy ordered.
B 650 10 10 molL. Measurements have been made to determine the solubilities of ethane C2H6 propane C3H8 and carbon dioxide CO2 in aqueous solutions of sodium cumene sulfonate NaCS at 25 degrees C. Why ethanol is more soluble in water than ethane.
Answer Ethanol is polar. Why is ethanol soluble in water. Solubility of Ethanol in Water Explain why ethanol CH3CH2OH is more soluble in water than is ethane CH3CH3.
Answer 1 of 2. The solubilities measured for each gas satisfy Henrys law at all concentrations of NaCS. The longer the carbon chain in an alcohol is the lower the solubility in polar solvents and the higher the solubility in nonpolar solvents.
Ethanol is more soluble in water because the hydroxyl group on the end of alcohol forms hydrogen bonds with water moleculeswhile ethane is hydrophobic and causes the solution to be highy ordered. Some alcohols are soluble in water due to the hydroxyl group which is polar hydrophilic. Due to presence of O-H group in ethanol it forms intermolecular H-bonding with highly polar solvent water.
Dissolution of ethanol gives rise to a AH while dissolution of ethane gives rise to AH n Ethanol is nonpolar while ethane is polar. This makes it possible to overcome the IMAFs of water. Ethanol is more soluble in water because the hydroxyl OH group on the end of the alcohol can form Hydrogen bonds with the water molecules where ethane is hydrophobic and causes the solution to.
Ethanol is more soluble in water than ethane due to the fact that ethanol has hydrogen bonding. General Organic and Biochemistry 1st Edition Edit edition Solutions for Chapter 9 Problem 53CQ. They do not dissolve in water.
The energy of hydration offsets this energy input in the case of ethanol but not ethene. The ethanol-OH group can hydrogen-bond with water. Technically ethanol should be LESS soluble in water than methanol.
The ethanol -OH group can hydrogen bond with water. Whereas ethanes weak london dispersion bonds would not be able to overcome waters IMAFs making it insoluble. The solubilities of C2H.
The amino group of. Why is ethanol more soluble in water than ethane is. Ethanol is polar Ethane is not.
Why is ethanol CH3CH20H more soluble in water than is ethane CH3CH3. Water and surface tension is going to be based on the force of confusion between the molecules or the attraction between the mo Limited Time Offer Unlock a free month of Numerade by answering 20 questions on our new app StudyParty. Ethanol is soluble in water because the polarity of its hydroxyl group is stronger than the nonpolarity of its two carbon chain.
This is due to the combined strength of so many hydrogen bonds forming between oxygen atoms of one alcohol molecule and the hydroxy H atoms of another. Hence ethanol is more soluble in water than ethyl ethanoate. As an example methane is soluble in ethanol diethyl ether benzene toluene methanol acetone.
In alcohols with more than five carbons in their chain the repulsive forces between the nonpolar chain and polar water do not allow the two to mix according to Solubility of Things. In other words ethanol is soluble in water because it is a polar solvent. It takes energy to take molecules of the substance dissolved appart.
The ethanol OH group can hydrogen-bond with water. Furthermore the melting and boiling temperatures of ethanol are comparatively higher than that of ethane because ethanol can form strong bonds such as hydrogen bonds between molecules which. Because it does have a higher polarity compared with ethane.
The dissolving of ethanol is thus energetically favored at large amounts whereas ethane-water mixtures tend to be most stable when in separation. Some organic compounds are soluble only in organic solvents. Dissolution of ethanol gives rise to a AS while dissolution of ethane gives rise to AS.
Solubility of Ethanol in WaterExplain why ethanol CH 3CH 2OH is more soluble in water than is ethane CH 3CH 3. James Armstrong Rent Buy. Ethanol is soluble in water primarily because of the presence of -OH group that allows or enables it to form hydrogen bonds with water molecules.
Q 1 Ethanal Is More Soluble In Water Than Ethane But Less Than The Ethanol Q 2 Acetone Is Completely Miscible In Water While Acetophenone Does Not
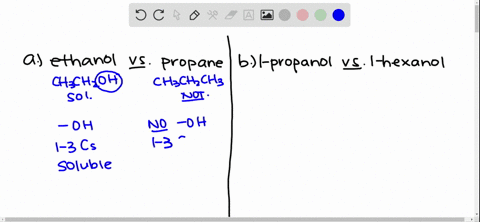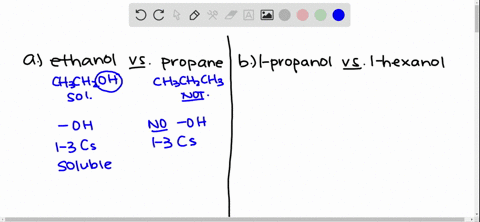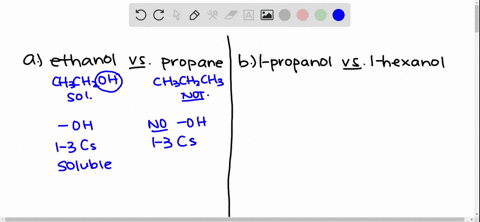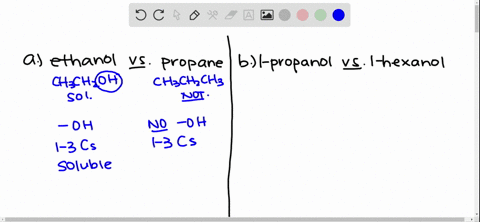
Solved Give An Explanation For The Following Observations A Methanol Is Soluble In Water But Ethane Is Not B 2 Propanol Is Soluble In Water But 1 Butanol Is Only Slightly Soluble

Solved Problem 1 Explain Why Ethanol Ch Ch Oh Is More Chegg Com
0 Comments